When I was an undergraduate I was often told by lecturers that I should find quantum mechanics very difficult, because it is unlike the classical physics I had learned about up to that point. The difference – or so I was informed – was that classical systems were predictable, but quantum systems were not. For that reason the microscopic world could only be described in terms of probabilities. I was a bit confused by this, because I already knew that many classical systems were predictable in principle, but not really in practice. I blogged about this some time ago, in fact. It was only when I had studied theory for a long time – almost three years – that I realised what was the correct way to be confused about it. In short, quantum probability is a very strange kind of probability that displays many peculiarities and subtleties that one doesn’t see in the kind of systems we normally think of as “random”, such as coin-tossing or roulette wheels.
To illustrate how curious the quantum universe is we have to look no further than the very basic level of quantum theory, as formulated by the founder of wave mechanics, Erwin Schrödinger. Schrödinger was born in 1887 into an affluent Austrian family made rich by a successful oilcloth business run by his father. He was educated at home by a private tutor before going to the University of Vienna where he obtained his doctorate in 1910. During the First World War he served in the artillery, but was posted to an isolated fort where he found lots of time to read about physics. After the end of hostilities he travelled around Europe and started a series of inspired papers on the subject now known as wave mechanics; his first work on this topic appeared in 1926. He succeeded Planck as Professor of Theoretical Physics in Berlin, but left for Oxford when Hitler took control of Germany in 1933. He left Oxford in 1936 to return to Austria but fled when the Nazis seized the country and he ended up in Dublin, at the Institute for Advanced Studies which was created especially for him by the Irish Taoiseach, Eamon de Valera. He remained there happily for 17 years before returning to his native land at the University of Vienna. Sadly, he became ill shortly after arriving there and died in 1961.
Schrödinger was a friendly and informal man who got on extremely well with colleagues and students alike. He was also a bit scruffy even to the extent that he sometimes had trouble getting into major scientific conferences, such as the Solvay conferences which are exclusively arranged for winners of the Nobel Prize. Physicists have never been noted for their sartorial elegance, but Schrödinger must have been an extreme case.
The theory of wave mechanics arose from work published in 1924 by de Broglie who had suggested that every particle has a wave somehow associated with it, and the overall behaviour of a system resulted from some combination of its particle-like and wave-like properties. What Schrödinger did was to write down an equation, involving a Hamiltonian describing particle motion of the form I have discussed before, but written in such a way as to resemble the equation used to describe wave phenomena throughout physics. The resulting mathematical form for a single particle is
 ,
,
in which the term  is called the wave-function of the particle. As usual, the Hamiltonian
is called the wave-function of the particle. As usual, the Hamiltonian  consists of two parts: one describes the kinetic energy (the first term on the right hand side) and the second its potential energy represented by
consists of two parts: one describes the kinetic energy (the first term on the right hand side) and the second its potential energy represented by  . This equation – the Schrödinger equation – is one of the most important in all physics.
. This equation – the Schrödinger equation – is one of the most important in all physics.
At the time Schrödinger was developing his theory of wave mechanics it had a rival, called matrix mechanics, developed by Werner Heisenberg and others. Paul Dirac later proved that wave mechanics and matrix mechanics were mathematically equivalent; these days physicists generally use whichever of these two approaches is most convenient for particular problems.
Schrödinger’s equation is important historically because it brought together lots of bits and pieces of ideas connected with quantum theory into a single coherent descriptive framework. For example, in 1911 Niels Bohr had begun looking at a simple theory for the hydrogen atom which involved a nucleus consisting of a positively charged proton with a negatively charged electron moving around it in a circular orbit. According to standard electromagnetic theory this picture has a flaw in it: the electron is accelerating and consequently should radiate energy. The orbit of the electron should therefore decay rather quickly.
Bohr hypothesized that special states of this system were actually stable; these states were ones in which the orbital angular momentum of the electron was an integer multiple of Planck’s constant. This simple idea endows the hydrogen atom with a discrete set of energy levels which, as Bohr showed in 1913, were consistent with the appearance of sharp lines in the spectrum of light emitted by hydrogen gas when it is excited by, for example, an electrical discharge. The calculated positions of these lines were in good agreement with measurements made by Rydberg so the Bohr theory was in good shape. But where did the quantised angular momentum come from?
The Schrödinger equation describes some form of wave; its solutions 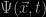 are generally oscillating functions of position and time. If we want it to describe a stable state then we need to have something which does not vary with time, so we proceed by setting the left-hand-side of the equation to zero. The hydrogen atom is a bit like a solar system with only one planet going around a star so we have circular symmetry which simplifies things a lot. The solutions we get are waves, and the mathematical task is to find waves that fit along a circular orbit just like standing waves on a circular string. Immediately we see why the solution must be quantized. To exist on a circle the wave can’t just have any wavelength; it has to fit into the circumference of the circle in such a way that it winds up at the same value after a round trip. In Schrödinger’s theory the quantisation of orbits is not just an ad hoc assumption, it emerges naturally from the wave-like nature of the solutions to his equation.
are generally oscillating functions of position and time. If we want it to describe a stable state then we need to have something which does not vary with time, so we proceed by setting the left-hand-side of the equation to zero. The hydrogen atom is a bit like a solar system with only one planet going around a star so we have circular symmetry which simplifies things a lot. The solutions we get are waves, and the mathematical task is to find waves that fit along a circular orbit just like standing waves on a circular string. Immediately we see why the solution must be quantized. To exist on a circle the wave can’t just have any wavelength; it has to fit into the circumference of the circle in such a way that it winds up at the same value after a round trip. In Schrödinger’s theory the quantisation of orbits is not just an ad hoc assumption, it emerges naturally from the wave-like nature of the solutions to his equation.
The Schrödinger equation can be applied successfully to systems which are much more complicated than the hydrogen atom, such as complex atoms with many electrons orbiting the nucleus and interacting with each other. In this context, this description is the basis of most work in theoretical chemistry. But it also poses very deep conceptual challenges, chiefly about how the notion of a “particle” relates to the “wave” that somehow accompanies it.

To illustrate the riddle, consider a very simple experiment where particles of some type (say electrons, but it doesn’t really matter; similar experiments can be done with photons or other particles) emerge from the source on the left, pass through the slits in the middle and are detected in the screen at the right.
In a purely “particle” description we would think of the electrons as little billiard balls being fired from the source. Each one then travels along a well-defined path, somehow interacts with the screen and ends up in some position on the detector. On the other hand, in a “wave” description we would imagine a wave front emerging from the source, being diffracted by the screen and ending up as some kind of interference pattern at the detector. This is what we see with light, for example, in the phenomenon known as Young’s fringes.
In quantum theory we have to think of the system as being in some sense both a wave and a particle. This is forced on us by the fact that we actually observe a pattern of “fringes” at the detector, indicating wave-like interference, but we also can detect the arrival of individual electrons as little dots. Somehow the propensity of electrons to arrive in positions on the screen is controlled by an element of waviness, but they manage to retain some aspect of their particleness. Moreover, one can turn the source intensity down to a level where there is only every one electron in the experiment at any time. One sees the dots arrive one by one on the detector, but adding them up over a long time still yields a pattern of fringes.
Curiouser and curiouser, said Alice.
Eventually the community of physicists settled on a party line that most still stick to: that the wave-function controls the probability of finding an electron at some position when a measurement is made. In fact the mathematical description of wave phenomena favoured by physicists involves complex numbers, so at each point in space at time  is a complex number of the form
is a complex number of the form  , where
, where 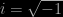 ; the corresponding probability is given by
; the corresponding probability is given by  . This protocol, however, forbids one to say anything about the state of the particle before it measured. It is delocalized, not being definitely located anywhere, but only possessing a probability to be any particular place within the apparatus. One can’t even say which of the two slits it passes through. Somehow, it manages to pass through both slits. Or at least some of its wave-function does.
. This protocol, however, forbids one to say anything about the state of the particle before it measured. It is delocalized, not being definitely located anywhere, but only possessing a probability to be any particular place within the apparatus. One can’t even say which of the two slits it passes through. Somehow, it manages to pass through both slits. Or at least some of its wave-function does.
I’m not going to into the various philosophical arguments about the interpretation of quantum probabilities here, but I will pass on an analogy that helped me come to grips with the idea that an electron can behave in some respects like a wave and in others like a particle. At first thought, this seems a troubling paradox but it only appears so if you insist that our theoretical ideas are literal representations of what happens in reality. I think it’s much more sensible to treat the mathematics as a kind of map or sketch that is useful for us to do find our way around nature rather than confusing it with nature itself. Neither particles nor waves really exist in the quantum world – they’re just abstractions we use to try to describe as much as we can of what is going on. The fact that it doesn’t work perfectly shouldn’t surprise us, as there are are undoubtedly more things in Heaven and Earth than are dreamt of in our philosophy.
Imagine a mediaeval traveller, the first from your town to go to Africa. On his journeys he sees a rhinoceros, a bizarre creature that is unlike anything he’s ever seen before. Later on, when he gets back, he tries to describe the animal to those at home who haven’t seen it. He thinks very hard. Well, he says, it’s got a long horn on its head, like a unicorn, and it’s got thick leathery skin, like a dragon. Neither dragons nor unicorns exist in nature, but they’re abstractions that are quite useful in conveying something about what a rhinoceros is like.
It’s the same with electrons. Except they don’t have horns and leathery skin. Obviously.



 Incidentally, there is a prominent relic of the Spanish and Portuguese Jews who settled in the East End right next to
Incidentally, there is a prominent relic of the Spanish and Portuguese Jews who settled in the East End right next to 










